Concentrated sulfuric acid is one of the most widely used chemicals in the chemical industry, but it is also one of the most unforgiving when it comes to equipment design.
Unlike water or common solvents, sulfuric acid demands special attention to corrosion behavior, material compatibility, moisture control, and flow conditions. A design that looks acceptable on paper can quickly become unsafe if these fundamentals are overlooked.
This article summarizes practical design considerations for tanks, piping, and pumps handling 98% sulfuric acid, based on real plant experience.
It is written especially for junior and early-career engineers, bridging the gap between textbook knowledge and actual plant design decisions.
Sulfuric acid is often the first “non-water” fluid that engineers face after gaining basic experience. Understanding its unique behavior is a critical step toward becoming a reliable process or mechanical engineer.
1. Process Flow Assumptions
Consider a typical sulfuric acid handling system:
- Storage tank: 10 m³
- Pump flow rate: 0.1 m³/min
- Fluid: 98% sulfuric acid
- Temperature: ambient
Tank size depends on consumption rate and delivery cycle, while pump capacity usually follows plant design standards (typical velocity limits). These assumptions form the basis for safe mechanical design.
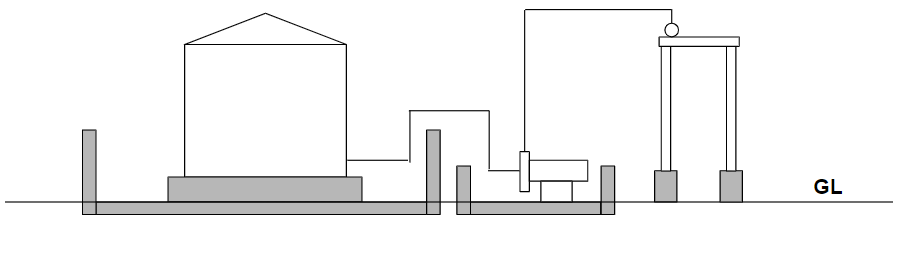
2. Tank Design Considerations
Material Selection
Metal tanks are generally preferred for concentrated sulfuric acid.
For tanks around 10 m³, glass-lined vessels are possible, but carbon steel (SS400) or stainless steel (SUS304) are more economical and practical.
- SS400: Suitable for concentrated sulfuric acid, but vulnerable when dilution occurs
- SUS304: Usable for concentrated and very dilute sulfuric acid, but unsafe in intermediate concentration ranges
For strictly concentrated acid, performance differences are limited.
The real risk arises when dilution occurs due to moisture ingress.
For this reason, SUS304 is generally the safer long-term choice, budget permitting.
3. Liquid Hold-Up and Moisture Control
Liquid accumulation must be minimized.
In outdoor tanks, standard designs are acceptable, but special attention is required at:
- Vapor space
- Weld joints between roof and shell
Any sulfuric acid trapped in the vapor zone can absorb moisture from air, producing dilute sulfuric acid, which dramatically increases corrosion risk.
4. Vent and Gas Lines
Vent lines should be designed to prevent rainwater and moisture ingress.
- Avoid upward-facing open vents
- Use 180-degree downward elbows instead
However, this can introduce air ingress during pumping operations. Over long periods, this increases dilution risk.
In some facilities, dry air or nitrogen sealing is applied to vent lines. While not always necessary, it should be considered for critical systems.
5. Dip Pipes and Internal Feed Design
Installing a dip pipe inside the tank is strongly recommended.
Feeding sulfuric acid from the top directly onto the liquid surface increases:
- Acid mist formation
- Contact with atmospheric moisture
- Risk of localized dilution
Feeding near the tank bottom reduces these risks.
However, high-velocity impingement requires wear plates.
For carbon steel tanks, this is especially critical because:
- SS400 relies on a protective corrosion film
- High velocity can strip this film and accelerate corrosion
This is one reason why SUS304 is often preferred for concentrated sulfuric acid systems.
6. Pump Selection
Pump Type
Seal-less pumps are strongly recommended.
- Centrifugal pumps with seals introduce water risks
- Canned motor pumps or magnetic drive pumps are suitable
- Canned motor pumps are generally the safest choice
⚠️ Avoid carbon bearings, which can fail rapidly in sulfuric acid service.
Pump Capacity and Hydraulic Design
Pressure loss calculations must not be treated casually.
Sulfuric acid properties at 20°C:
- Density: ~1840 kg/m³
- Viscosity: ~26 cP
These values are far from water-like.
Pipe diameters often need to be one size larger than for water or solvents to maintain acceptable velocities and losses.
7. Piping Design
Material
- SUS304 is the safest default choice
- Carbon steel piping (SGP or STPG) is risky, even with increased wall thickness
If you are unsure, choose stainless steel.
Bolting materials generally do not require special upgrades; carbon steel bolts are acceptable.
Connections
- Flanged connections are preferred
- JIS 10K flanges are often sufficient
- Threaded joints should be avoided due to leakage risk
Gaskets
- Compressed fiber sheet gaskets are generally acceptable
- PTFE gaskets are also suitable in many cases
Conclusion
Designing equipment for concentrated sulfuric acid requires more than selecting corrosion-resistant piping.
Safe systems depend on:
- Proper material selection
- Moisture exclusion
- Velocity control
- Realistic hydraulic calculations
Sulfuric acid systems involve tanks, piping, pumps, valves, and sometimes heat exchangers and agitators.
Among all factors, temperature effects and corrosion behavior have the greatest influence on safety and equipment life.
Junior engineers should never design sulfuric acid systems in isolation.
Consult experienced engineers, review operating history, and always incorporate feedback from the field.
Careful, conservative design is not overengineering—it is professional responsibility.
About the Author – NEONEEET
A user‑side chemical plant engineer with 20+ years of end‑to‑end experience across design → production → maintenance → corporate planning. Sharing practical, experience‑based knowledge from real batch‑plant operations. → View full profile

Comments